The Levenberg Lab specializes in the field of stem cell-based tissue engineering. Our focus is on recreating complex tissues in vitro for purposes such as regenerative medicine, disease modeling, and drug discovery. We have expertise in engineering 3D vascularized composite tissues using cutting-edge 3D bioprinting and scaffold-based approaches. By utilizing defined biomaterials and mimicking in vivo settings with mechanical signals, we investigate the mechanisms behind in vitro vascularization. In addition, our research extends to exosome-mediated spinal cord regeneration and the emerging field of cultivated meat.
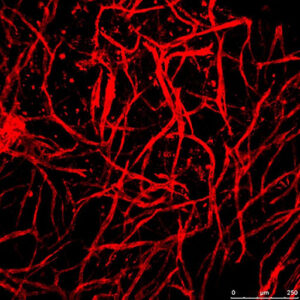
Engineering blood vessel networks
In this project we explored the effects of mechanical forces, scaffold type and supporting cells on angiogenesis. We examined different endothelial cells and support cells under various conditions, with a particular focus on how engineered vessels align in response to mechanical forces, and integrate in-vivo.
This study was supported by the FP7 ERC starting Grant, Engvasc.
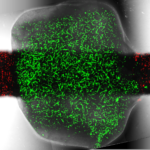
Engineering Composite Tissues for Facial Reconstruction
This study is supported by the Horizon 2020 ERC Consolidator Grant, VesselNet.
For more information, please visit the The ERC VesselNet Project web page
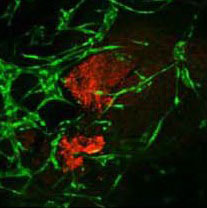 3D Bioprinting of Pancreatic Tissue
3D Bioprinting of Pancreatic Tissue
This study is supported by Horizon 2020, FET-Open – Novel ideas for radically new technologies, Pan3D.
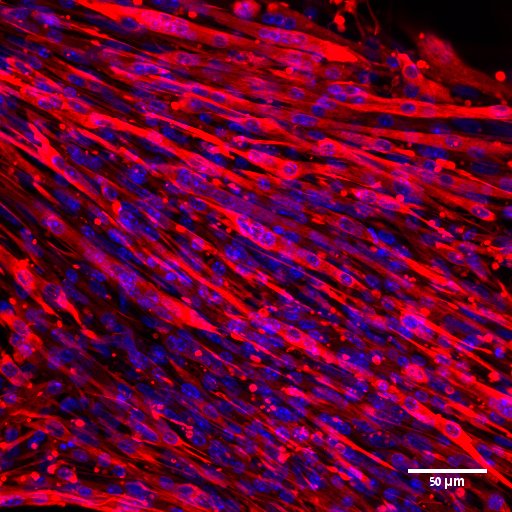 Restoring Insulin Sensitivity in Type 2 Diabetes Patients using Engineered Muscle Tissue
Restoring Insulin Sensitivity in Type 2 Diabetes Patients using Engineered Muscle Tissue
This study is supported by the The Rina and Avner Schneur Center of Diabetes Research
(http://schneur-diabetic-center.net.technion.ac.il/)
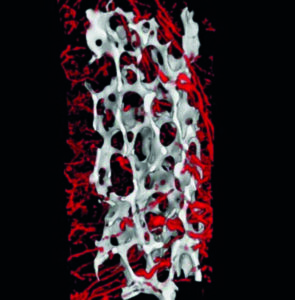
Bone Tissue Repair
The main hurdle in bone tissue repair is maintaining appropriate vascularization for regeneration of large-scaled defects. We fabricate engineered constructs, capable of soft and hard tissue repair. These pre-vascularized composite grafts are fabricated from FDA approved and biocompatable biomaterials, which undergo an induction phase by differential seeding with diverse cellular components. Our bone tissue engineering (BTE) models are treated from a clinical perspective, utilizing in vivo imaging and CAD-CAM interfaces to produce defect-specific grafts. These engineered constructs are implanted in live animal models to assess their ability to regenerate complex anatomical structures.
This study is supported by the Israel science foundation.

Spinal Cord Injury Regeneration
Stem cell-based therapies hold great potential to treat spinal cord injury and additional nervous system traumatic syndromes due to lack of regeneration in the adult nervous system. Our research focuses on delivering stem cells into a complete spinal cord injury. We utilize tissue engineering methods to enhance stem cell integration and survival post implantation. In addition we study the effect of vascularization on spinal cord regeneration. Alternatively, we exploit the regenerative effects of stem cell-derived exosomes as therapeutics and drug delivery platforms to target the spinal cord lesion.
This study was supported by the J&J Shervington Fund (SL), and the Israel Foundation for Spinal Cord Injury (SL).
 Cardiac Muscle Regeneration using a Perfusable Cardiac Patch
Cardiac Muscle Regeneration using a Perfusable Cardiac Patch
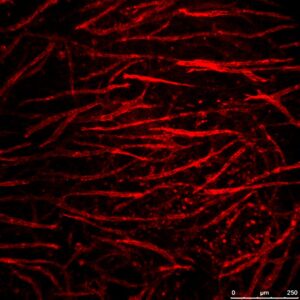
Geometric and Mechanical Patterning of Hierarchical Vascular Networks
This research focuses on exploring the biophysical factors controlling vascular architecture, remodeling, and integration upon implantation. Using cutting edge methods to pattern the organization of cells, and to map and control mechanical forces within the 3D constructs, the role of cell-generated forces, initial endothelial cell organization and external mechanical forces in the location, orientation and extent of sprouting is studied. In addition, the impact of hierarchical network geometry, rationally designed, on implant integration in-vivo is assessed.
This research is supported by United States-Israel BSF grant in collaboration with Prof. Christopher Chen from Boston University.
 Effect of scaffold geometry on vascular networks
Effect of scaffold geometry on vascular networks
Many environmental factors can affect the behavior of newly sprouting vessels. Among them, scaffold geometry provides mechanical cues that are translated into chemical and physical cell signaling, which impacts on the decisions made by migrating vessels. This research studies how different scaffold geometries can help control the behavior and orientation of sprouting vessels during network formation.
This research is supported by funding from the University of Michigan – Israel Partnership for Research.

Cell-Grown Meat
The aim of this project is to produce meat from cell cultures, using tissue engineering techniques. We isolate stem cells from a bovine origin, expand them, and seed them on 3D scaffolds, to develop bovine skeletal muscle tissues.
We study food-related properties of bovine skeletal muscle tissues under different cell combinations, scaffolds and media composition.
This study is supported by Aleph Frams (Aleph Farms)
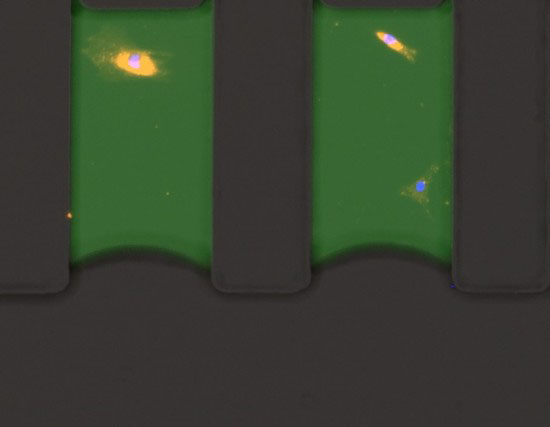 Nano-liter Microfluidic device
Nano-liter Microfluidic device
The company site Nanosynex

